
Understanding the Classification of Diamonds
Understanding the diamond types makes you a better diamond expert
When diamond crystals form deep inside the earth there are other elements present that can become a part of the diamond crystal. The two with the greatest impact are nitrogen (N) and boron (B).
Nitrogen (N) is the most common you will find and is the main factor of off-color yellow diamonds as well as fancy yellow-colored diamonds. This is because the nitrogen atoms will absorb certain wavelengths of light thereby causing the transmitted color of light leaving the diamond to be something other than white or colorless.
An example of the above is the Type 1a diamond, (sometimes written as Type Ia with Roman numeral I). In this diamond type the nitrogen absorbs the blue color of light. As a result, Type Ia diamonds will have a strong tendency to offer a slightly yellowish body color.
Let's take a look at the four main types of diamonds. We are only going to take it down to these four basic types for our purposes here. You may hear of even smaller divisions of diamond types based on the number of nitrogen atoms in the crystal lattice. While this may have scientific importance, for our purposes of learning diamond quality grading the four divisions as listed below are the main divisions and the ones you will hear about most often in the market.
Type I Diamonds
Type I diamonds will have nitrogen present in the carbon crystal lattice. These diamonds compose the vast majority of diamonds on the market, and the variations of color will be directly impacted by the manner in which the nitrogen is present in Type I diamonds.
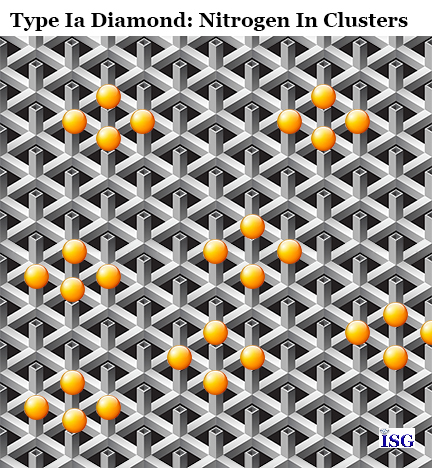
Type Ia diamonds have nitrogen atoms that exist as clumps within the diamond's carbon crystal lattice structure. Type Ia diamonds compose almost 98% of the diamonds found. The nitrogen atom groups have a tendency to absorb the blue light wavelength, thereby producing tinted yellow and brown color diamonds. At left you see a graphic showing how the nitrogen atoms form in clumps in the diamond crystal lattice. It should be noted that this is not a true rendition of the real diamond crystal lattice, but is presented here to help you remember the various types of diamonds and what goes into qualifying each within that group.
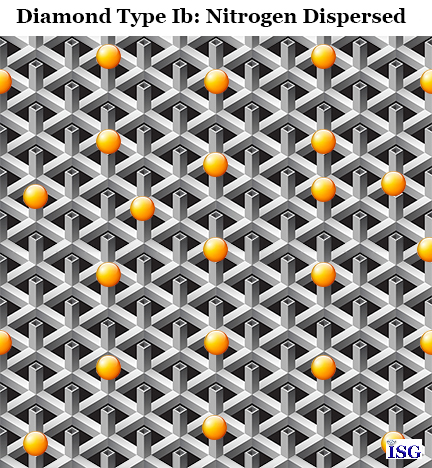
Type Ib diamonds will still have nitrogen, but instead of residing as clumps of nitrogen atoms as Type Ia, Type Ib diamonds will have nitrogen atoms residing as single atoms interspersed throughout the diamond crystal lattice. This allows for a more uniform and complete presence of nitrogen in the diamond crystal, and for a remarkably different color impact on the diamond itself.
The impact of the single nitrogen atoms of Type Ib diamonds is that they now absorb more of the blue and green colors. Thereby causing a more intense and therefore deeper fancy yellow color. True natural Canary Yellow diamonds are most often of Type Ib, owing to the fact that the nitrogen absorbs a greater wavelength of colors from the yellow and blue spectrum, leaving a more pure yellow color, which is why most of your fancy yellow diamonds will indeed be Type Ib diamonds?
It should be noted that less than 1/10th of 1% of diamonds found are of Type Ib, which is one reason that fancy yellow-colored diamonds are so rare and valuable.
Above is another graphic to demonstrate how the nitrogen atoms are interspersed within the carbon diamond crystal. And again, this is not out of a physics textbook, it's only so you can get a visual concept of the difference in a Type Ia and a Type Ib diamond in relation to the presence of nitrogen atoms in each.
Type II Diamonds
Type II diamonds will be virtually free from the presence of nitrogen. These diamonds are considered the purest of all diamonds owing to the fact that there are very few if any, impurities in their crystal structure.
_______________________________________________________________________________________
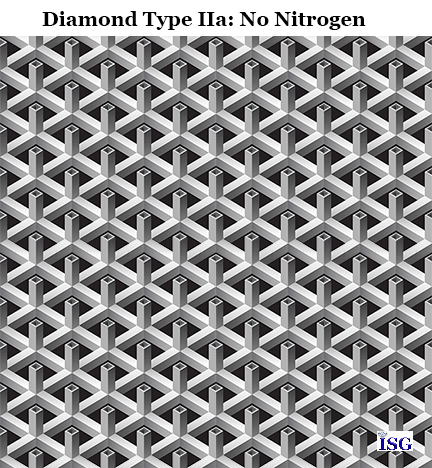
Type IIa diamonds are the purest of all of the diamond types. These diamonds contain virtually no nitrogen…and most often none at all. These diamonds are generally completely colorless since only carbon is present in their crystal structure. It should be noted that most of your really big and famous diamonds such as the Koh-i-Noor diamond and others are of Type IIa. That is because these diamonds are of the purest formation and grow quite large, and they are the most transparent and colorless since they have no nitrogen in their crystal lattice structure.
At left is a graphic of the carbon structure to help you remember that the Type IIa diamonds are the purest crystal and usually produce a pure colorless crystal. Between 1 and 2 percent of diamonds on the market are of Type IIa diamonds.
_______________________________________________________________________________________
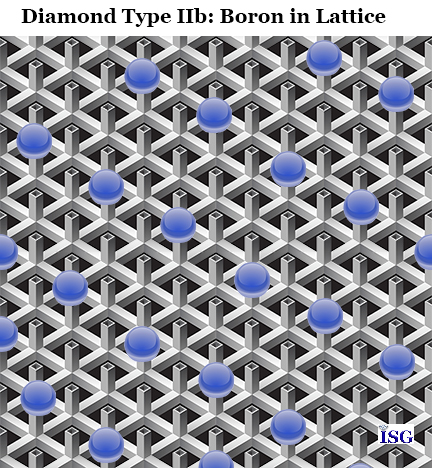
Type IIb diamonds are truly rare and unusual. With a Type IIb, you have an impurity of boron (B) in the crystal structure. This has two important impacts on the diamond.
First, it causes the diamond to be electrically conductive, something no other diamond type will offer. In fact, the other classifications of diamond will be electrical insulators rather than conductors. So the presence of boron in the diamond crystal lattice will cause the diamond to be an electrical conductor.
Second, it causes the diamond to have a natural blue color, and sometimes a blue-grey color. This is how the famous blue Hope Diamond formed, as a result of the presence of boron in the diamond crystal. These natural blue diamonds are extremely rare and extremely expensive, owing to the fact that less than 1/10th of 1 percent of diamonds found are natural blue.
The key here is that the two factors serve a single and very important purpose: Natural blue diamonds are very expensive. However, there are radiated blue diamonds on the market that are artificially colored. These are fairly common in the current market and are valued far below a natural blue Type IIb diamond.
Owing to the fact that only natural blue diamonds are electrical conductors, it is fairly easy to identify a blue diamond as being natural or irradiated by testing for electrical conductivity.
This makes natural blue Type IIb diamonds both unique and valuable.
We hope everyone is hanging in there with this Covid-19 mess going on. Stay strong and stay active. This will all be over soon. We will stay with you all the way.
Robert James FGA, GG
President, International School of Gemology
Property and Casualty Adjuster, Texas Department of Insurance #1300433
Member: National Association of Independent Insurance Adjusters
We welcome you to join us for the most fun and rewarding study of gemology and jewelry appraisal in the world....and at the most affordable price. Visit the ISG and learn about our programs.
https://schoolofgemology.com/
©2020 International School of Gemology. ALL RIGHTS RESERVED. We encourage sharing and caring throughout the industry as long as all copyrights are left intact.
ISG
International School of Gemology
PO Box 1727
Helotes, TX 78023